ਈਥੇਨੋਲ
ਦਿੱਖ
(ਈਥੇਨਾਲ ਤੋਂ ਮੋੜਿਆ ਗਿਆ)
ਫਰਮਾ:Chembox pKbਫਰਮਾ:Chembox Bioavailਫਰਮਾ:Chembox Metabolismਫਰਮਾ:Chembox Metabolitesਫਰਮਾ:Chembox HalfLifeਫਰਮਾ:Chembox Excretionਫਰਮਾ:Chembox Legal statusਫਰਮਾ:Chembox Dependence liability
| ਈਥੇਨੋਲ | |
|---|---|
|
|
|

|
ethanol[1] | |
Other names Absolute alcohol, alcohol, cologne spirit, drinking alcohol, ethylic alcohol, EtOH, ethyl alcohol, ethyl hydrate, ethyl hydroxide, ethylol, grain alcohol, hydroxyethane, methylcarbinol | |
| Identifiers | |
| CAS number | 64-17-5 |
| PubChem | 702 |
| ChemSpider | 682 |
| UNII | 3K9958V90M |
| DrugBank | DB00898 |
| IUPHAR ligand | 2299 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
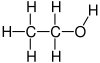
| ਅਣਵੀ ਫ਼ਾਰਮੂਲਾ | C2H6O |
| ਮੋਲਰ ਭਾਰ | 46.07 g mol−1 |
| ਦਿੱਖ | Colorless liquid |
| ਘਣਤਾ | 0.789 g/cm3 (at 20°C) |
| ਪਿਘਲਨ ਅੰਕ |
−114 °C, 159 K, -173 °F |
| ਉਬਾਲ ਦਰਜਾ |
78.37 °C, 352 K, 173 °F |
| ਘੁਲਨਸ਼ੀਲਤਾ in water | miscible |
| log P | −0.18 |
| ਵਾਸ਼ਪੀ ਦਬਾਅ | 5.95 kPa (at 20°C) |
| ਤੇਜ਼ਾਬਪਣ (pKa) | 15.9 (H2O), 29.8 (DMSO)[2][3] |
| ਅਪਵਰਤਿਤ ਸੂਚਕ (nD) | 1.361 |
| ਲੇਸ | 1.2 mPa·s (at 20°C), 1.074 mPa·s (at 25°C)[4] |
| ਡਾਈਪੋਲ ਮੋਮੈਂਟ | 1.69 D[5] |
| Pharmacology | |
| ATC code | D08 |
| Routes of administration |
Common: oral Uncommon: suppository, inhalation, ocular, insufflation,[8] injection[9] |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
ਈਥੇਨਾਲ ਇੱਕ ਪ੍ਰਸਿਧ ਅਲਕੋਹਲ ਹੈ।
ਹਵਾਲੇ
[ਸੋਧੋ]- ↑ ਹਵਾਲੇ ਵਿੱਚ ਗ਼ਲਤੀ:Invalid
<ref>tag; no text was provided for refs namedPubchem - ↑ Ballinger, P., Long, F.A.; Long (1960). "Acid Ionization Constants of Alcohols. II. Acidities of Some Substituted Methanols and Related Compounds1,2". Journal of the American Chemical Society. 82 (4): 795–798. doi:10.1021/ja01489a008.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Arnett, E.M., Venkatasubramaniam, K.G.; Venkatasubramaniam (1983). "Thermochemical acidities in three superbase systems". J. Org. Chem. 48 (10): 1569–1578. doi:10.1021/jo00158a001.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Lide, David R., ed. (2012). CRC Handbook of Chemistry and Physics (92 ed.). Boca Raton, FL.: CRC Press/Taylor and Francis. pp. 6–232.
- ↑ Lide, David R., ed. (2008). CRC Handbook of Chemistry and Physics (89 ed.). Boca Raton: CRC Press. pp. 9–55.
- ↑ Swift, Robert (December 2003). "Direct measurement of alcohol and its metabolites". Addiction. 98: 73–80. doi:10.1046/j.1359-6357.2003.00605.x. PMID 14984244. Retrieved 26 March 2015.
- ↑ 7.0 7.1 ਹਵਾਲੇ ਵਿੱਚ ਗ਼ਲਤੀ:Invalid
<ref>tag; no text was provided for refs namedpmid5457514 - ↑ Stogner, John M.; Eassey, John M.; Baldwin, Julie Marie; Miller, Bryan Lee (September 2014). "Innovative alcohol use: Assessing the prevalence of alcohol without liquid and other non-oral routes of alcohol administration". Drug and Alcohol Dependence. 142: 74–78. doi:10.1016/j.drugalcdep.2014.05.026. PMID 25012895. Retrieved 26 March 2015.
- ↑ Gilman, Jodi M; Ramchandani, Vijay A; Crouss, Tess; Hommer, Daniel W (28 September 2011). "Subjective and Neural Responses to Intravenous Alcohol in Young Adults with Light and Heavy Drinking Patterns". Neuropsychopharmacology. 37 (2): 467–477. doi:10.1038/npp.2011.206. PMID 21956438. Retrieved 26 March 2015.
- ↑ WHO Expert Committee on Problems Related to Alcohol Consumption : second report (PDF). Geneva, Switzerland: World Health Organization. 2007. p. 23. ISBN 9789241209441. Retrieved 3 March 2015.
...alcohol dependence (is) a substantial risk of regular heavy drinking...
